Live
- Synergy Marine Group Champions Onboard AI Agriculture for Healthier Crews and a Greener Future
- BJP alleges Rs 45 cr scam by AAP govt in school construction
- Yashoda Medicity organizes “Crown of Courage” ceremony to honor Cancer Survivors
- AAP appoints two-time legislator Arora as Punjab chief
- BJP won’t succeed in toppling Congress govt: K’taka Home Minister
- Turkey neutralises five Kurdish militants in Syria
- BJP questions Kejriwal on 21,000 deaths in Delhi due to contaminated water
- CM Revanth Reddy Inspects Progress of Telangana Mother Statue Installation
- ‘It’s Congress habit to spread lies’, says Vinod Tawde after sending legal notice to Kharge, Rahul
- Hungarian PM invites Netanyahu for visit, calls ICC warrants 'brazen and cynical'
Just In
DRDO Project Director and Scientist Dr Sudhir Chandana
At a time when the world is struggling with trial-and-error method to treat the novel Coronavirus and the new mutants causing great concern, the news that DRDO had developed an anti-Covid oral drug, 2-deoxy-D-glucose (2DG) in collaboration with Dr Reddy's lab comes as the best news of the century and boosts the confidence level of all Covid-19 patients
At a time when the world is struggling with trial-and-error method to treat the novel Corona virus and the new mutants causing great concern, the news that DRDO had developed an anti-Covid oral drug, 2-deoxy-D-glucose (2DG) in collaboration with Dr Reddy's lab comes as the best news of the century and boosts the confidence level of all Covid-19 patients. The drug is likely to be available for the patients in about a week's time. In an exclusive interview with Purna Singh, DRDO Project Director and Scientist Dr Sudhir Chandana explains how the drug with no side effects would work on the patients.
India is waiting with a bated breath for the rollout of the oral drug for Covid-19. The news that it would be rolled out soon gives great courage to people. But when will it be available for the Covid affected people?
Dr Reddy's Lab is our industrial partner. They have already geared up their manufacturing process. We got emergency approval for the use of this drug. We can expect it to be on the floor within one week for the masses. Although the final status of production can be explained by Reddy's Lab.
When did DRDO start clinical trials and how does this drug work? Is it just an immunity booster like a vaccine or a drug to cure Covid-19? Does it stop the mutation of the virus, or does it make the virus less effective?
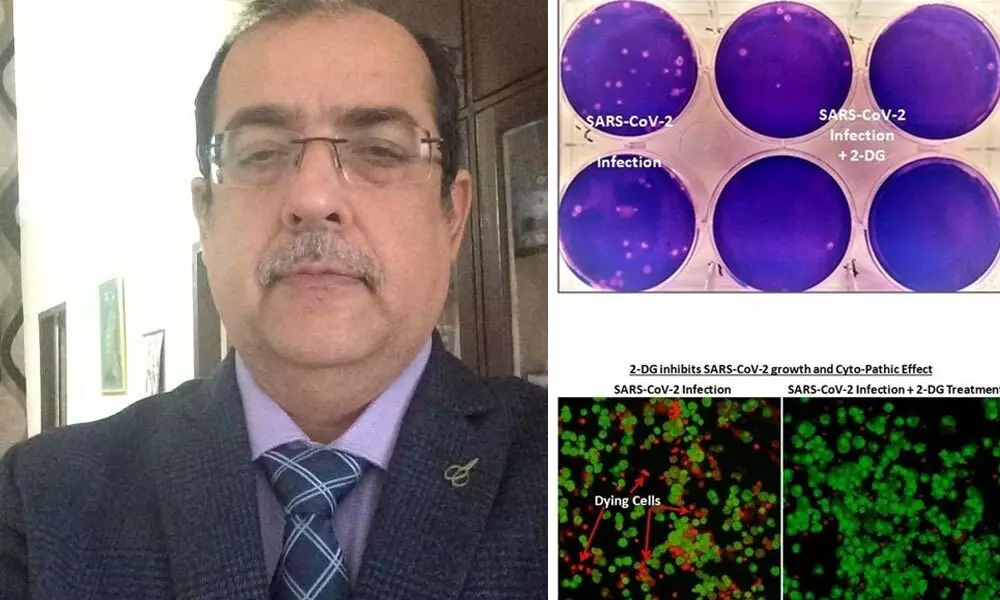
The clinical trials had started from May 2020 and in October 2020 we went in for phase 2 trials. The phase 3 trials started from December 2020 and went on till March 2021. The mechanism of this molecule is that it is a glucose analog. The virus-infected cell, the host cells are more dependent on glycolysis, and the glucose intake increases. 2DG is competing with glucose for uptake by the cell. So, the virus-infected cells take more of 2DG also which in turn leads to a higher intake of 2DG in the cells. Eventually, it inhibits the energy production in the virus cells. This finally results in replication or multiplication of the virus. This we have already established by in-vitro experiments and the efficacy and the effectiveness of the drug in the cell was quite good. In simple language, it reduces dependence on oxygen.
When can the doctors administer this drug to the patient? Will the drug be able to cut down the number of patients in the ICU by saving their lives?
We have conducted trials of this drug on 110 patients on oxygen supply in the third phase of the trials. All of them recovered pretty well. There was no death. In the phase 2 trials, we have seen that the recovery is faster and then in the larger sample size in phase 3 trials we could see that by third-day oxygen dependence reduces in more patients as compared to standard care. This drug has been administered along with standard care.
Are there any side effects of the drug either in the short or long run?
No side effects were evident during trials. It is pretty safe drug to be administered.
What are the other chemicals needed to synthesise the drug? Will the same be easily available in our country?
Yes. This is made from glucose, so anyone can start preparing it. Only the process thing is the issue with each company and Dr Reddy's lab had already collaborated with us and the previous trials of 2G were on brain tumour patient from 2004 onwards up to 2014 and they had also taken technology from DRDO Gwalior and made the process patent for the synthesis of the molecule. Dr Reddy's Lab has the Terms of Trade (ToT)
Everybody is eyeing a drug that would be saviour for mankind. Can we expect this drug to be one of the so-called "Ram-baan" drug to cure Covid?
The kind of response we have seen in the clinical trials, definitely indicates that it has given good benefits in the recovery of patients. In both phase 2 and 3 trials, we have seen one indication that in none of the patients the disease was progressing further. All have recovered. So we can imply that this drug along with other standard care is giving benefits. And no patient has lost his life, so this is a very good indication. People have been calling it "Ram- Baan" but we would say it is a very good drug.

© 2024 Hyderabad Media House Limited/The Hans India. All rights reserved. Powered by hocalwire.com







