Live
- Yadadri temple trust discloses financial report
- Karimnagar: Social media deciding fate of netas and their parties
- Challa Vamsichand Reddy gains support at Shadnagar meeting
- Bhadrachalam: A grand celebration of spirituality and tradition
- April 27 last date for final payment for Haj-2024
- Veerlapalli Shankar leads Mudiraj Community’s support rally for CM
- Irrigation projects: BRS fires failure salvo against TG govt
- New Bhadrachalam bridge over Godavari set to open
- Top officials of TS, AP hold joint meet to ensure hassle-free polls
- Congress’ farmers declaration and guarantees are bogus: Kishan Reddy
Just In
Changing schedule of doses can improve malaria vaccine efficacy

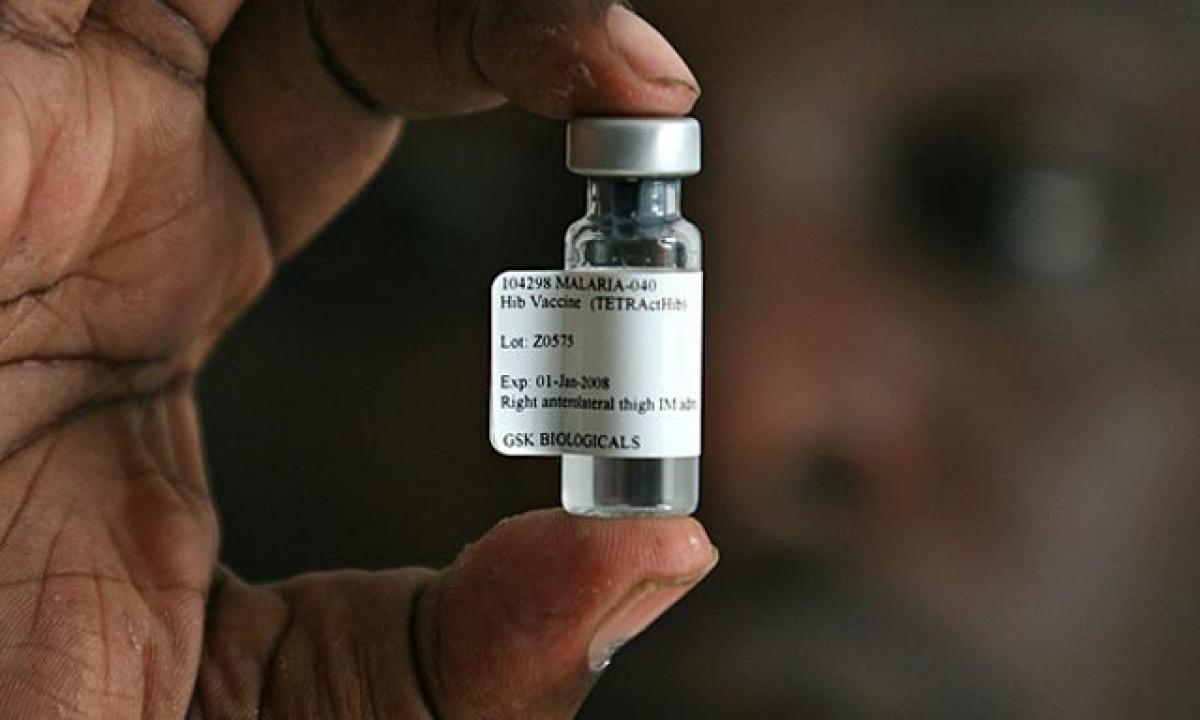
Researchers at the Walter Reed Army Institute of Research (WRAIR) have shown that the efficacy of malaria vaccine candidate, RTS,S/AS01, can be improved to approximately 87 per cent, compared with 63 per cent using the current standard regimen.
Researchers at the Walter Reed Army Institute of Research (WRAIR) have shown that the efficacy of malaria vaccine candidate, RTS,S/AS01, can be improved to approximately 87 per cent, compared with 63 per cent using the current standard regimen.
This study was conducted in a small number of malaria-naïve adults in the US, using the controlled human malaria infection model (CHMI), also referred to as the malaria challenge model.
"Although absolute antibody concentration did not increase with greater prevention of malaria, we were able to demonstrate that this new regimen has a definite impact to improve antibody function in humans," said lead author Jason Regules.
"We hope that further improvements in the efficacy of RTS,S/AS01 are within reach," Regules said. The RTS,S/AS01 malaria vaccine candidate - developed by GSK in the early 1980s and, since 2001, in partnership with the global health non-profit PATH for use in young children - has successfully completed a pivotal large-scale phase three trial in Africa.
WRAIR's human malaria infection model also showed the first safety and efficacy of this malaria vaccine in humans. But earlier studies had shown that the vaccine's ability to protect against malaria waned over time.
In light of the partial efficacy profile of RTS,S/AS01, scientists at WRAIR and partner organisations have continued investigating a variety of strategies to improve the absolute level of the vaccine's efficacy.
In this study, published in the Journal of Infectious Diseases, researchers sought to determine if a novel immunisation schedule would significantly increase the candidate vaccine's ability to protect against infection.
As part of the study, the researchers immunised study participants according to two regimens - a zero-, one-, seven-month schedule with a fractional third dose (Fx017M) and the current standard zero-, one-, two-month schedule (012M).
Following the third vaccination, study participants were exposed to malaria-causing parasites. Twenty-six of 30 study participants (86.7 per cent) who received the new regimen and 10 of 16 (62.5 per cent) who received the standard regimen were protected from infection following the first CHMI.
The new regimen delayed infection longer when compared to the standard regimen, as well. Study participants did not report any serious health events as a result of receiving the vaccinations, and no safety concerns were associated with reducing dosages, the study said.

© 2024 Hyderabad Media House Limited/The Hans India. All rights reserved. Powered by hocalwire.com






