Live
- TG seeks PMAY fund boost as 80pc of State turns urban
- Don’t buy assets from known ganja smugglers: DIG
- Youth Cong takes out ‘mashaal rally’ condemning Amit Shah’s statement on Ambedkar
- Hyderabad remained peaceful in 2024,but sees sharp spike in cybercrimes
- MP, West MLA distribute cheques to critical patients
- Hyderabad Book Fair a big draw among book lovers
- GVMC secures 1st place in best public awareness programme category
- 39 transgenders recruited as Traffic Assistants
- Jesus’ teachings promote unity, compassion: Haryana Governor
- Special drive held on road safety
Just In
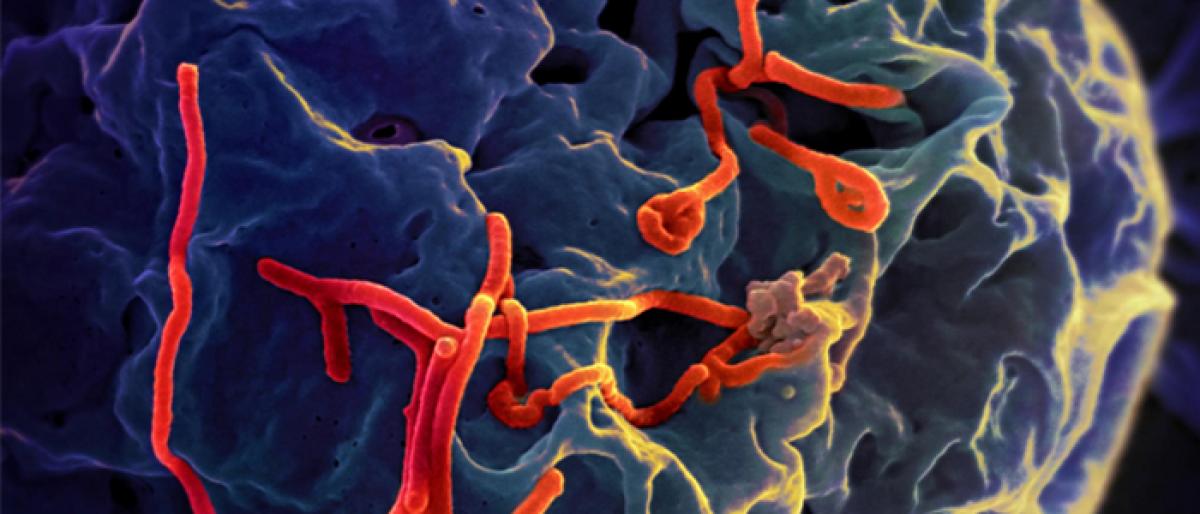
Scientists, including one of Indianorigin, have found that the novel synthetic DNA vaccine is safe against Ebola virus and offers a longterm alternative to traditional vaccines
New York: Scientists, including one of Indian-origin, have found that the novel synthetic DNA vaccine is safe against Ebola virus and offers a long-term alternative to traditional vaccines.
The team, from The Wistar Institute in Philadelphia, US, optimised a shorter, dose-sparing, immunisation regimen and simplified vaccine that can be directly administered into the skin. They targeted a virus surface protein called glycoprotein.
This new approach induced rapid and protective immunity from virus challenges.
Importantly, the approach showed strong immune responses one year after the last dose, supporting the long-term immunogenicity of the vaccine -- a particularly challenging area for Ebola vaccines.
"Synthetic non-viral based DNA technology allows for rapid vaccine development by delivery directly into the skin, resulting in consistent, potent and rapid immunity compared to traditional vaccine approaches," said lead researcher David B. Weiner, Director of Wistar's Vaccine and Immunotherapy Center.
"An anti-Ebola virus DNA vaccine like this may provide an important new tool for protection, and we are excited to see what future studies will unveil," he added.
In the study, published in the Journal of Infectious Diseases, the team detected antibody levels were equal or higher to those reported for other vaccines currently being evaluated in the clinic.
"The success of intradermal delivery of a low-dose regimen is very encouraging," said Ami Patel, Ph.D., associate staff scientist in the Weiner Lab. "The ultimate goal of our work is to create effective and safe vaccines that are optimised for field use in at-risk areas."
Ebola virus disease is a serious and often fatal illness that can cause fever, headache, muscle pain, weakness, fatigue, diarrhoea, vomiting, stomach pain and haemorrhage (severe bleeding).
First discovered in humans in 1976 in the Democratic Republic of the Congo, the largest outbreak occurred in West Africa from 2014 to 2016, which claimed more than 11,000 lives, according to the World Health Organization.
The death rate is about 50 per cent and the virus is spread by contact with contaminated body fluids, including blood and semen.
While there are no licensed treatments available for Ebola virus disease yet, multiple experimental therapies are being developed.

© 2024 Hyderabad Media House Limited/The Hans India. All rights reserved. Powered by hocalwire.com







