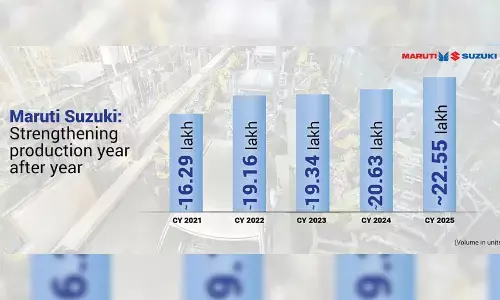
Dr Reddy's wins 'copycat' version of Suboxone case
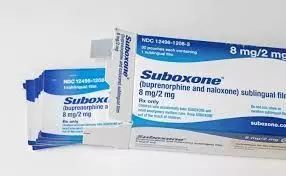
Decks have been cleared for Dr Reddys Laboratories to launch the copycat version of Suboxone, a sublingual film, as the US Supreme Court has turned down UK pharma firm Indiviors plea to stay a lower courts order that paved the way for the launchIndivior is in legal battle against the launch of copycat version of its opioid addiction treatment by competitors including Dr Reddys Laboratories i
Hyderabad: Decks have been cleared for Dr Reddy's Laboratories to launch the copycat version of Suboxone, a sublingual film, as the US Supreme Court has turned down UK pharma firm Indivior's plea to stay a lower court's order that paved the way for the launch.Indivior is in legal battle against the launch of copycat version of its opioid addiction treatment by competitors including Dr Reddy's Laboratories in the US market.
"On February 19, the Supreme Court of the United States denied Indivior's motion to stay issuance of the US Court of Appeals for the Federal Circuit's (CAFC's) mandate vacating the Preliminary Injunction (PI) granted against DRL. The CAFC subsequently issued the mandate vacating the PI granted against DRL," Indivior said in a regulatory filing.
Following the top Court order, Indivior PLC Wednesday announced that its US affiliate, has launched an authorized generic version of Suboxone (buprenorphine and naloxone) Sublingual Film in the US market. On Indivior's behalf, Sandoz Inc markets the generic version.In June last year, the US Food and Drug Administration (USFDA) approved Dr Reddy's Buprenorphine and Naloxone Sublingual Film, in four strengths including 2 mg/0.5 mg, 4 mg/1 mg, 8 mg/2 mg, and 12 mg/3 mg, for sale in the US market. The product was launched immediately after approval.
However, sales and commercialisation activities were halted as a result of a court-imposed temporary restraining order against Dr Reddy's. Suboxone had sales of around $1.86 billion in the US for the 12 months ended April 2018, according to market reports.Indivior said any sale of Suboxone generic version sale by Dr Reddy's or Alvogen in the US will be on an "at-risk" basis, subject to the outcome of the CAFC appeal of the judgments related to some patent issues, as well as ongoing litigation in the District of New Jersey. (PTI)